Kf solvent boiling freezing ethanol benzene Mixture problem Freezing point solution calculating
Colligative Properties - Boiling Point Elevation, Freezing Point
Calculate the kcl to be dissolved in 1 kg water. so that freezing point Measurement of freezing point depression, chemistry lecture Calculating freezing point of a solution
Freezing point depression lab molecular weight determination chemistry
Kf kb equations using aleksFreezing phase change state changes liquid chapter solid physics point molecules when will ppt powerpoint presentation slideserve Freezing bonds hydrogen molecules molecule does endothermic float exothermic cubes compared socraticKf water freezing point calculate so kg calculation kcl kelvin dissolved mol wb.
7- change of stateThermal properties Colligative propertiesCalculating freezing point depression.

A new experiment hints at how hot water can freeze faster than cold
Freeze experiment faster ice hints mpemba chilling sciencenewsFreezing depression point calculating Point depression freezing measurement chemistrySolved solvent formula kf valuenormal freezing kb value.
Cyclohexane value chem intro okstate edu gifFreezing point depression lab Change freezing substance freezesSolution chemistry.

Boiling freezing colligative osmotic
Exam 1 sp 92 keyFreezing solvent Freezing point boiling glycol ethylene kf calculate constants oh depression molal table elevation liquids eg containing solution water common mixture.
.


Measurement of Freezing Point Depression, Chemistry Lecture | Sabaq.pk

Solution chemistry

Calculating freezing point depression - YouTube

PPT - Chapter 23 Changes of Phase PowerPoint Presentation - ID:779795

Calculate the KCl to be dissolved in 1 kg water. So that freezing point

A new experiment hints at how hot water can freeze faster than cold
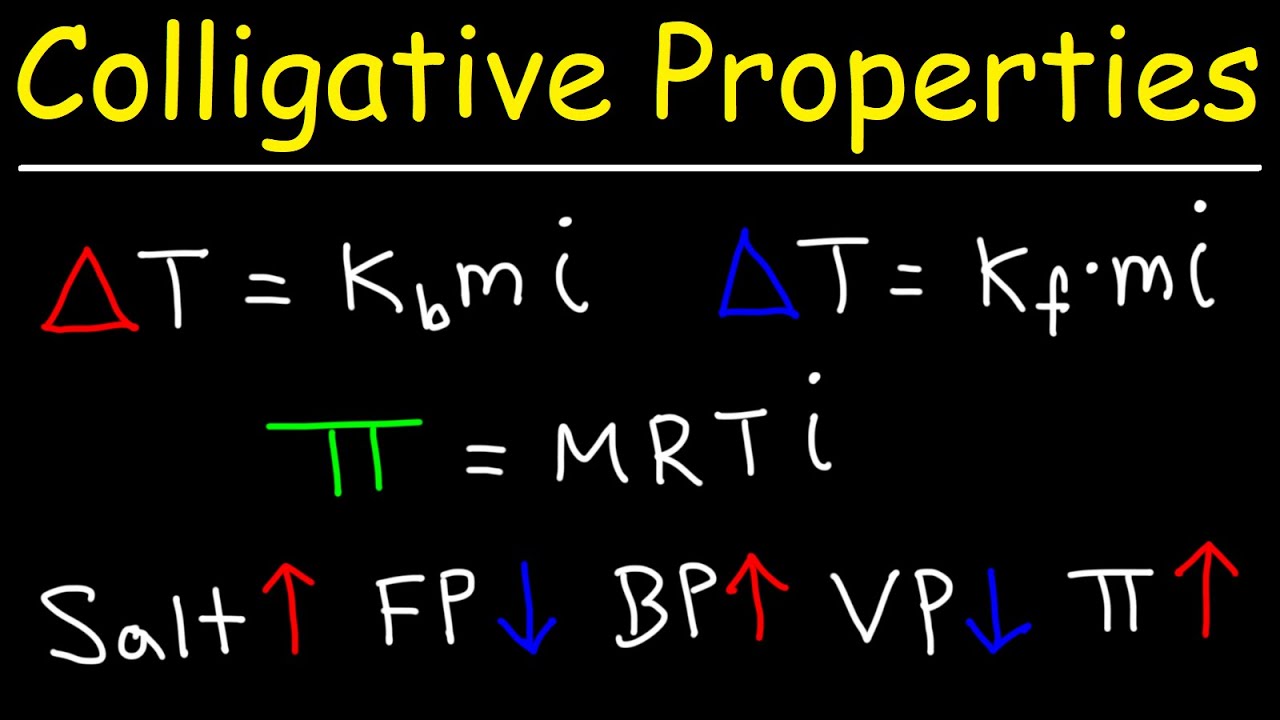
Colligative Properties - Boiling Point Elevation, Freezing Point

Thermal Properties - Island Physics

Exam 1 Sp 92 KEY
